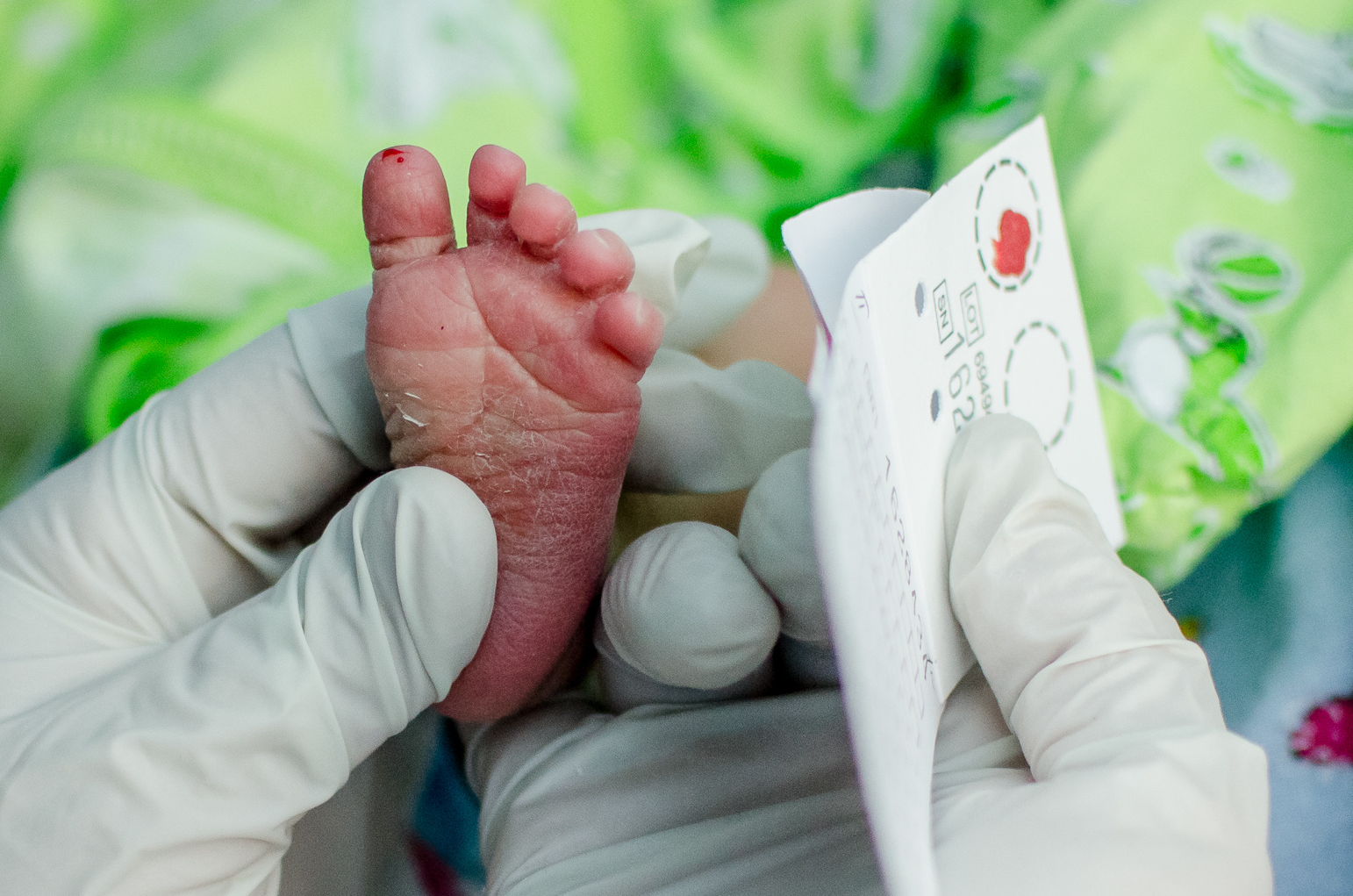
PPMD’s Newborn Screening Pilot recently completed two years of ground-breaking recruitment. More than 36,000 families consented to participate and more than 36,000 babies have been screened for Duchenne Muscular Dystrophy in New York State. As a result of screening, four babies with Duchenne or Becker and one carrier female were diagnosed and provided with optimal early care. The pilot program utilized PPMD’s tools, resources, and expertise and the Newborn Screening Translational Research Network (NBSTRN) made up of partners from the National Institute of Child Health and Human Development, National Institutes of Health (NICHD/NIH) program housed at the American College of Medical Genetics and Genomics (ACMG), and the New York State Department of Health.
The pilot is a major step in the process of bringing newborn screening (NBS) for Duchenne and Becker to all families, as a prospective pilot is required in a nomination for the Recommended Uniform Screening Panel (the RUSP). Data from the pilot is in the process of being analyzed. They are looking at information provided by families who participated, as well as trying to understand the most accurate way to screen babies in the future.
We are grateful to our Industry partners who helped to fund the pilot; PTC Therapeutics, Sarepta Therapeutics, PerkinElmer, Pfizer, Inc., Solid Biosciences and Wave Life Sciences. The pilot was led by a consortium of Duchenne industry partners with a commitment to early diagnosis and intervention in Duchenne and guided by a Steering Committee of representatives from federal agencies, provider groups, and representatives from key Duchenne stakeholder communities.
PPMD has been working with a number of workgroups of experts in Duchenne and newborn screening to gather all the resources and data needed to complete the RUSP nomination, which includes understanding the condition, the available treatments, and the pilot. We are incredibly grateful to all of our workgroup and steering committee participants for their willingness to share their time and expertise:
| Ms. Natasha Bonhomme | Dr. Erik Henricson | Dr. Michele Puryear |
| Ms. Beth Boyea | Ms. Annie Kennedy | Dr. Eddie Smith |
| Dr. Amy Brower | Ms. Carrie Koval-Burt | Ms. Natalie Street |
| Dr. Michele Caggana | Dr. Kate Kucera | Dr. Norma Tavakoli |
| Dr. Wendy Chung | Dr. Jerry Mendell | Dr. David Tegay |
| Dr. Emma Ciafaloni | Dr. Kristie Mercer | Dr. Tracy Trotter |
| Dr. Anne Connolly | Dr. Max Muenke | Dr. Bob Vogt |
| Ms. Mardee DeSantis | Dr. Stan Nelson | Dr. Michael Watson |
| Dr. Kevin Flanigan | Dr. Garey Noritz | Ms. Julia Wynn |
| Dr. Dorota Gruber | Dr. Holly Peay |
With five approved therapies – and a research pipeline filled with potential therapeutic interventions – NBS will eliminate the long diagnostic odyssey and enable early treatment. While we still have much work to be done, the completion of this pilot is a crucial step.



 by: Parent Project Muscular Dystrophy
by: Parent Project Muscular Dystrophy

